First in-human trial of novel therapy was presented at the American Heart Association’s Scientific Sessions 2023

Findings from a phase 1 trial reported by a Cleveland Clinic physician show that a single dose of an experimental therapy produced greater than 94% reductions in blood levels of lipoprotein(a), a key driver of heart disease risk, with the results lasting for nearly a year.
Results from the “Efficacy and Safety of Lepodisiran: An Extended Duration Short-Interfering RNA Targeting Lipoprotein(a) Study” were presented today during a late-breaking science session at the American Heart Association’s Scientific Sessions 2023 and simultaneously published in the Journal of the American Medical Association.
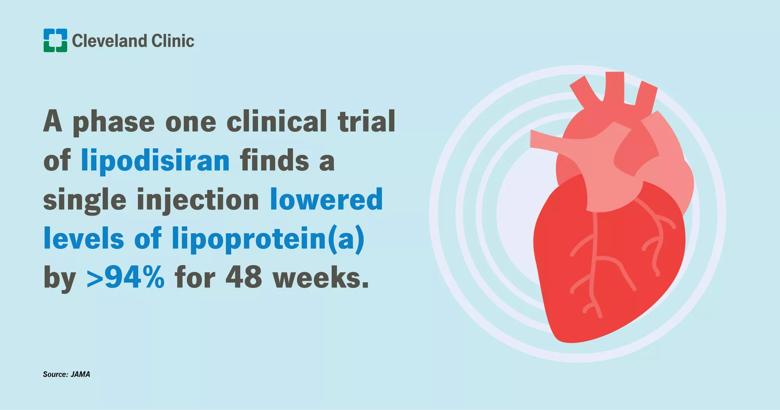
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/81b6bcea-edf2-4edc-bd71-d14bb543cb51/23-CCC-4338108-Lipodisiran-Phase-1-Study-FB-TW-10_27-1_jpg)
Lipoprotein(a), often shortened to just Lp(a), is made in the liver and has similarities to LDL, also known as low-density lipoprotein or “bad cholesterol.” Unlike other types of cholesterol particles, Lp(a) levels are 80-90% genetically determined. The structure of the Lp(a) particle causes the accumulation of plaque in arteries which greatly increases the risk of heart attacks and strokes.
Although effective therapies exist to reduce the risk of heart disease by lowering LDL cholesterol and other lipids, currently there are no approved drug treatments to lower Lp(a). Since Lp(a) levels are determined by a person’s genes, lifestyle changes (diet or exercise) have no effect.
In the trial, participants who received an injection of lepodisiran had lipoprotein(a) levels reduced by the top dose as much as 96% within two weeks and maintained levels more than 94% below baseline for 48 weeks. The drug is a small interfering RNA (siRNA) therapeutic that blocks the messenger RNA needed to manufacture a key component of lipoprotein(a) in the liver.
The findings add lepodisiran to the growing list of therapies that could be promising treatments for atherosclerotic cardiovascular diseases in people with high levels of Lp(a), which is estimated to affect 64 million people in the United States and 1.4 billion people worldwide.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/1d0b8db6-c98a-42b1-a699-d37182807610/nissen-steve-074-e1605303442379_jpg)
Steven E. Nissen, M.D.
“These results showed that this therapy was well tolerated and produced very long-duration reductions in Lp(a), an important risk factor that leads to heart attack, stroke and aortic stenosis,” said lead author Steven Nissen, M.D., Chief Academic Officer of the Heart, Vascular & Thoracic Institute at Cleveland Clinic.
In the trial, researchers enrolled 48 patients in the U.S. and Singapore with a mean age of 47. Investigators studied six different dosages and a placebo, which were all administered as injections. Participants were monitored for up to 48 weeks after administration.
Maximum Lp(a) plasma concentrations were reduced by 49% from baseline levels for the 4 mg dose and up to 96% for the 608 mg dose vs. a 5% decrease for the placebo. No safety issues were observed, and the only tolerability issue was mild injection site reactions.
“Despite the strong evidence of the importance of elevated Lp(a) as a risk factor for heart disease, effective treatment has been elusive,” said Dr. Nissen. “This approach to treatment gives hope to the 20% of the world’s population who have elevated Lp(a) levels.”
A phase 2 trial studying lepodisiran is currently underway. The trial was sponsored by Eli Lilly and Company (Lilly), the company developing lepodisiran.
Dr. Nissen has served as a consultant for many pharmaceutical companies and has overseen clinical trials for Amgen, AbbVie, AstraZeneca, Bristol Myers Squibb, Mineralys, New Amsterdam Pharmaceuticals, Eli Lilly and Company, and Novartis. However, he does not accept honoraria, consulting fees or other compensation from commercial entities.
Cleveland Clinic is a nonprofit multispecialty academic medical center that integrates clinical and hospital care with research and education. Located in Cleveland, Ohio, it was founded in 1921 by four renowned physicians with a vision of providing outstanding patient care based upon the principles of cooperation, compassion and innovation. Cleveland Clinic has pioneered many medical breakthroughs, including coronary artery bypass surgery and the first face transplant in the United States. Cleveland Clinic is consistently recognized in the U.S. and throughout the world for its expertise and care. Among Cleveland Clinic’s 82,600 employees worldwide are more than 5,786 salaried physicians and researchers, and 20,700 registered nurses and advanced practice providers, representing 140 medical specialties and subspecialties. Cleveland Clinic is a 6,728-bed health system that includes a 173-acre main campus near downtown Cleveland, 23 hospitals, 280 outpatient facilities, including locations in northeast Ohio; Florida; Las Vegas, Nevada; Toronto, Canada; Abu Dhabi, UAE; and London, England. In 2024, there were 15.7 million outpatient encounters, 333,000 hospital admissions and observations, and 320,000 surgeries and procedures throughout Cleveland Clinic’s health system. Patients came for treatment from every state and 112 countries. Visit us at clevelandclinic.org. Follow us at x.com/CleClinicNews. News and resources are available at newsroom.clevelandclinic.org.
Editor’s Note: Cleveland Clinic News Service is available to provide broadcast-quality interviews and B-roll upon request.