First-in-human trial of deep brain stimulation for stroke recovery has begun
Cleveland Clinic News Service | 216.444.0141
We’re available to shoot custom interviews & b-roll for media outlets upon request.
CCNS health and medical content is consumer-friendly, professional broadcast quality (available in HD), and available to media outlets each day.

Cleveland Clinic has performed the first deep brain stimulation (DBS) surgery for stroke recovery, as part of an ongoing clinical trial assessing the procedure’s potential to improve movement in patients recovering from stroke.
Stroke is the leading cause of long-term disabilities in the United States. Despite rehabilitative efforts, one-third of stroke patients maintain long-term motor deficits severe enough to be disabling.
A team led by Andre Machado, M.D., Ph.D., chairman of Cleveland Clinic’s Neurological Institute, performed the DBS surgery Dec. 19, 2016. During the 6 hour procedure, electrodes were implanted in a part of the patient’s brain called cerebellum, which has extensive connections with the cerebral cortex. Connected to a pace-maker device, DBS electrodes provide small electric pulses as a way to help people recover control of their movements.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/95b94bec-a1e4-418c-b586-ab86c99f3655/ndre-machado_jpeg)
Andre Machado, M.D., Ph.D., chairman of Cleveland Clinic’s Neurological Institute
“If this research succeeds, it is a new hope for patients that have suffered a stroke and have remained paralyzed after a stroke. It is an opportunity to allow our patients to rehabilitate and gain function and therefore gain independence,” Dr. Machado said. “Our knowledge to date shows that deep brain stimulation can help the brain reorganize, can help the brain adapt, beyond what physical therapy alone can do. The goal of our study is to boost rehabilitation outcomes beyond what physical therapy alone could achieve.”
Over the next few weeks, the patient – who has been discharged home feeling well and in stable condition – will continue to heal and recover from brain surgery, followed by physical therapy. After a few weeks of rehabilitation, the DBS device will be turned on as the patient continues physical therapy. The patient will be monitored and evaluated regularly to determine how DBS can boost the effects of physical therapy.
Video content: This video is available to watch online.
View video online (https://www.youtube.com/embed/Bnl28ZM5Kfw)
“In addition to characterizing the effect of treatment on motor recovery, we will examine directly how stimulation affects brain activity using a combination of non-invasive imaging and electrophysiological techniques,” said Kenneth Baker, Ph.D., of Cleveland Clinic’s Department of Neurosciences and co-primary investigator on the research grant. “Through these studies, we hope to gain further insight into its therapeutic mechanisms and, perhaps more importantly, how best to optimize delivery of the therapy as we move forward.”
Dr. Machado’s previous research has shown that DBS targeting the same brain pathway in a laboratory model promotes the brain’s plasticity, the ability to form new neural connections, during recovery from stroke. This clinical trial expands on that work and for the first time translates it to humans. This is currently an experimental approach and, as for any surgical procedure, has risks. Potential risks include hemorrhage, infection and neurological complications. Additional information about the trial can be found at https://clinicaltrials.gov/ct2/show/NCT02835443.
This first-in-human trial is co-funded by an NIH BRAIN Initiative Grant: Brain Research through Advancing Innovative Neurotechnologies and this is among one of many projects exploring human brain activity.
Dr. Machado patented the DBS method in stroke recovery. Boston Scientific owns a license to those patents and provided the Vercise DBS systems used in the trial. In 2010, Cleveland Clinic Innovations established a for-profit spin-off company, Enspire DBS Therapy to fund the clinical trial and commercialize the method; Dr. Machado holds stock options and equity ownership rights with Enspire and serves as the chief scientific officer. Boston Scientific recently invested $2.5 million into Enspire DBS.
Editor’s Note: Photos, videos and an animation are available for download below. An interview with Dr. Macahado and surgery video are available at the following links:
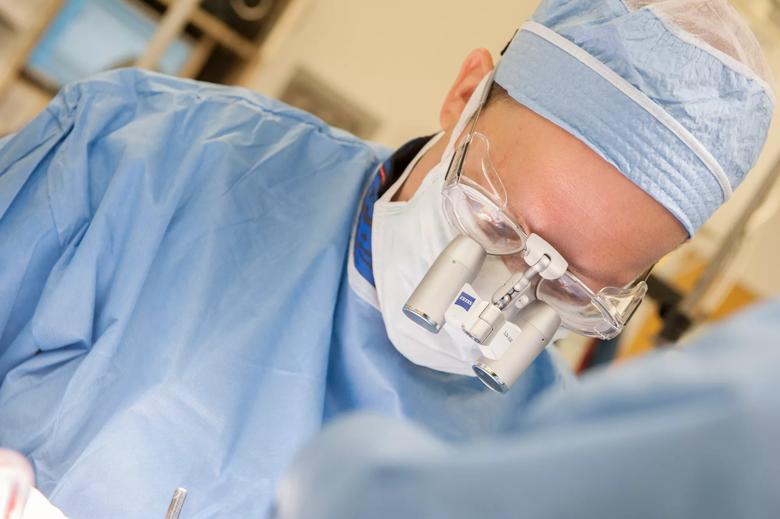
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/5717898a-bad3-44c6-af7e-bd4ddf54d2f5/Machado-DBS-087872_08-05-14_037_DG_jpg)
Andre Machado, M.D., Ph.D., chairman of Cleveland Clinic’s Neurological Institute, performs DBS surgery.
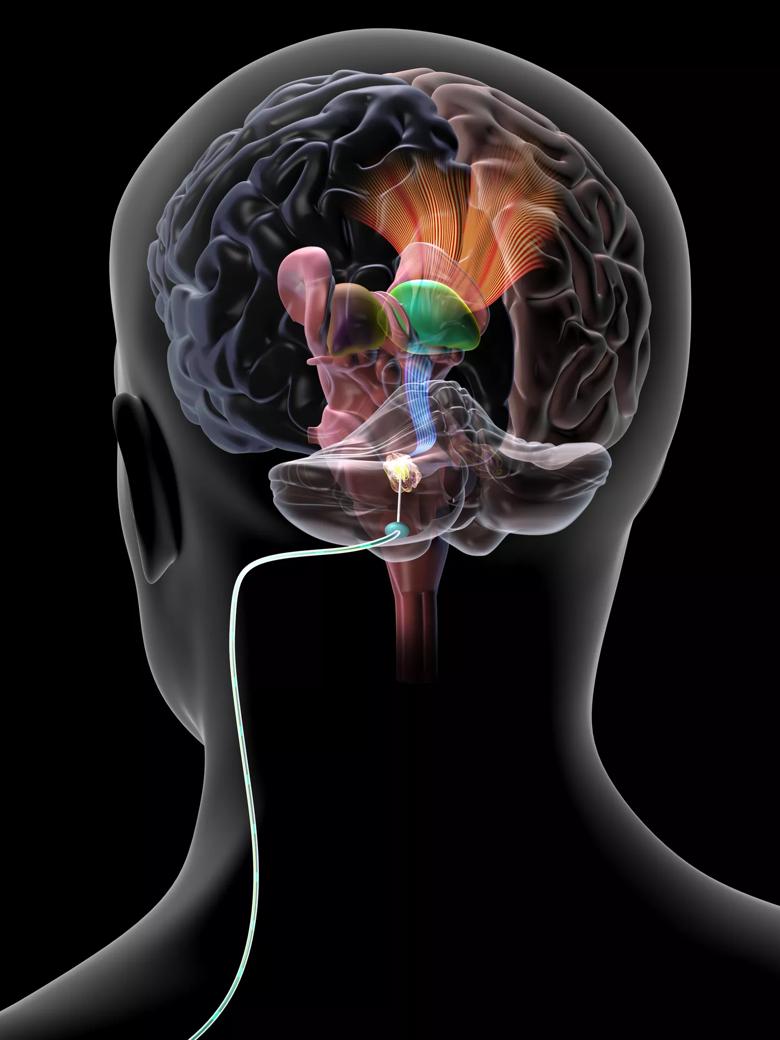
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/57099390-09eb-4104-8fd7-20605deed895/DBS-Cerebellar-DBS3_jpg)
Illustration of a deep brain stimulation lead implanted in the cerebellar dentate nucleus for treatment of post-stroke motor deficits.
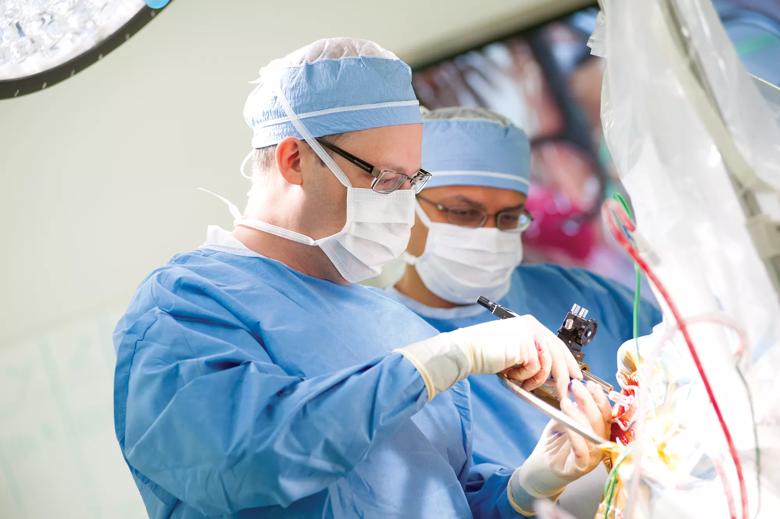
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/1580914e-6a7f-4522-af1c-0200907faee0/Machado-DBS-070418_06-03-13_0146_TM_jpg)
Andre Machado, M.D., Ph.D., chairman of Cleveland Clinic’s Neurological Institute, performs DBS surgery.
Video content: This video is available to watch online.
View video online (https://www.youtube.com/embed/EbJDJSQhxV8?feature=oembed&wmode=transparent)
Video content: This video is available to watch online.
View video online (https://www.youtube.com/embed/8jacQOSOCwc?feature=oembed&wmode=transparent)
Cleveland Clinic is a nonprofit multispecialty academic medical center that integrates clinical and hospital care with research and education. Located in Cleveland, Ohio, it was founded in 1921 by four renowned physicians with a vision of providing outstanding patient care based upon the principles of cooperation, compassion and innovation. Cleveland Clinic has pioneered many medical breakthroughs, including coronary artery bypass surgery and the first face transplant in the United States. Cleveland Clinic is consistently recognized in the U.S. and throughout the world for its expertise and care. Among Cleveland Clinic’s 82,600 employees worldwide are more than 5,786 salaried physicians and researchers, and 20,700 registered nurses and advanced practice providers, representing 140 medical specialties and subspecialties. Cleveland Clinic is a 6,728-bed health system that includes a 173-acre main campus near downtown Cleveland, 23 hospitals, 280 outpatient facilities, including locations in northeast Ohio; Florida; Las Vegas, Nevada; Toronto, Canada; Abu Dhabi, UAE; and London, England. In 2024, there were 15.7 million outpatient encounters, 333,000 hospital admissions and observations, and 320,000 surgeries and procedures throughout Cleveland Clinic’s health system. Patients came for treatment from every state and 112 countries. Visit us at clevelandclinic.org. Follow us at x.com/CleClinicNews. News and resources are available at newsroom.clevelandclinic.org.
Editor’s Note: Cleveland Clinic News Service is available to provide broadcast-quality interviews and B-roll upon request.