Bempedoic acid studied in nearly 14,000 patients in global study
Content is property of Cleveland Clinic and for news media use only.

Findings from a Cleveland Clinic-led clinical trial showed that the use of bempedoic acid (a cholesterol-lowering drug) in statin-intolerant patients lowered low-density lipoprotein (LDL) cholesterol and reduced heart attacks and coronary procedures.
Findings were presented today during a Late Breaking Science Session at the American College of Cardiology’s 72nd Annual Scientific Session in New Orleans and simultaneously published in the New England Journal of Medicine.
Elevated LDL cholesterol can accumulate in the walls of blood vessels, creating blockages and raising the risk of heart attack or stroke. Statins are the standard first-line treatment for the prevention of cardiovascular disease and work by lowering cholesterol levels in the blood. Statins have been shown in many studies to reduce the risk of stroke, heart attack and death when administered to patients, both with a history of cardiovascular disease and without. However, some patients struggle with adverse side effects like muscle pain, headaches or weakness that prevent them from using statins at recommended doses.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/1d0b8db6-c98a-42b1-a699-d37182807610/nissen-steve-074-e1605303442379_jpg)
Steven E. Nissen, M.D.
Bempedoic acid is approved by the U.S. Food and Drug Administration as an additional treatment to help lower cholesterol in patients with certain conditions who have high cholesterol despite maximally tolerated statin therapy. The trial, called CLEAR Outcomes, is the first to explore whether bempedoic acid could reduce cardiovascular outcomes.
The study enrolled 13,970 statin-intolerant patients between December 2016 and August 2019 at 1,250 sites in 32 countries. To participate, patients and their physicians were required to confirm that the patient could not tolerate statins. All participants had LDL-c levels of 100 mg/dL or higher and either a previous cardiac event or other risk factors for heart disease. Participants were randomly assigned to take 180 mg of bempedoic acid or a placebo daily and followed for an average of over 3 years. The primary outcome studied was a composite of cardiovascular death, heart attack, stroke or coronary revascularizations (a procedure to open blocked arteries).
The primary outcome was reduced 13% with bempedoic acid treatment. When different types of cardiac events were analyzed separately, bempedoic acid was found to reduce heart attacks by 23% and coronary revascularizations by 19%.
“Until now, there have not been any drugs designed specifically for statin-intolerant patients,” said the study’s lead author Steven E. Nissen, MD, chief academic officer of the Heart Vascular & Thoracic Institute at Cleveland Clinic. “While statins remain the cornerstone of risk reduction in patients with elevated LDL cholesterol, this is a major step forward for a population who need statins but suffer troublesome side-effects.”
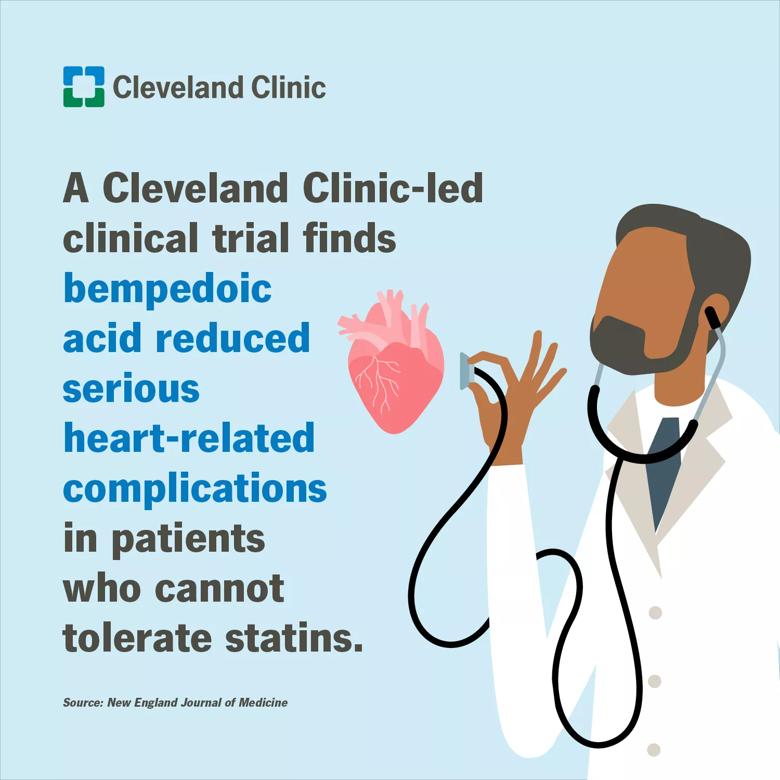
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/a2915189-18f8-4d88-bd8f-1514e93847f5/1500x1500-bempedoic-acid-graphic-cv-1_jpg)
Steven E. Nissen, M.D.
Bempedoic acid differs from statins by not activating until it reaches the liver. This limits the drug’s effects on muscle, or other tissues or organs, reducing the likelihood of side effects reported with statins.
Researchers note that the 20-25% reduction in LDL cholesterol reported for bempedoic acid is less than the 40-50% reductions typically achieved with statins. “Overall, these results reveal bempedoic acid can still make a significant difference in the risk of serious cardiac events for patients who cannot tolerate statins,” said Dr. Nissen.
The study was funded by Esperion Therapeutics, developer of bempedoic acid.
Dr. Nissen has served as a consultant for many pharmaceutical companies and has overseen clinical trials for AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Eli Lilly, Novartis, Mineralys, Silence Therapeutics, and Pfizer. However, he does not accept honoraria, consulting fees or other compensation from commercial entities.
Video content: This video is available to watch online.
View video online (https://www.youtube.com/embed/saWmgRA8F50?feature=oembed&wmode=transparent)
Clinical Trial Shows Drug for Statin-Intolerant Patients Reduces Serious Heart Complications
Cleveland Clinic is a nonprofit multispecialty academic medical center that integrates clinical and hospital care with research and education. Located in Cleveland, Ohio, it was founded in 1921 by four renowned physicians with a vision of providing outstanding patient care based upon the principles of cooperation, compassion and innovation. Cleveland Clinic has pioneered many medical breakthroughs, including coronary artery bypass surgery and the first face transplant in the United States. U.S. News & World Report consistently names Cleveland Clinic as one of the nation’s best hospitals in its annual “America’s Best Hospitals” survey. Among Cleveland Clinic’s 72,500 employees worldwide are more than 5,050 salaried physicians and researchers, and 17,800 registered nurses and advanced practice providers, representing 140 medical specialties and subspecialties. Cleveland Clinic is a 6,500-bed health system that includes a 173-acre main campus near downtown Cleveland, 21 hospitals, more than 220 outpatient facilities, including locations in northeast Ohio; southeast Florida; Las Vegas, Nevada; Toronto, Canada; Abu Dhabi, UAE; and London, England. In 2021, there were 10.2 million total outpatient visits, 304,000 hospital admissions and observations, and 259,000 surgical cases throughout Cleveland Clinic’s health system. Patients came for treatment from every state and 185 countries. Visit us at clevelandclinic.org. Follow us at twitter.com/ClevelandClinic. News and resources available at newsroom.clevelandclinic.org.
Editor’s Note: Cleveland Clinic News Service is available to provide broadcast-quality interviews and B-roll upon request.