First in-human trial phase one results published in Nature Medicine. The study was also supported by Enspire DBS Therapy, Inc.

Researchers at Cleveland Clinic have conducted a first-in-human trial of deep brain stimulation (DBS) for post-stroke rehabilitation patients, demonstrating that using DBS to target the dentate nucleus is safe and feasible. The dentate nucleus regulates fine-control of voluntary movements, cognition, language, and sensory functions in the brain. The trial, called EDEN, also showed that nine out of 12 participants demonstrated improvements in both motor impairment and function. The study, which was also supported by Enspire DBS Therapy, Inc., found that participants with at least minimal preservation of distal motor function at enrollment showed gains that almost tripled their initial scores. This research builds on more than a decade of preclinical work led by principal investigators Andre Machado, MD, PhD and Kenneth Baker, PhD at Cleveland Clinic. To learn more, read the full Cleveland Clinic news release.
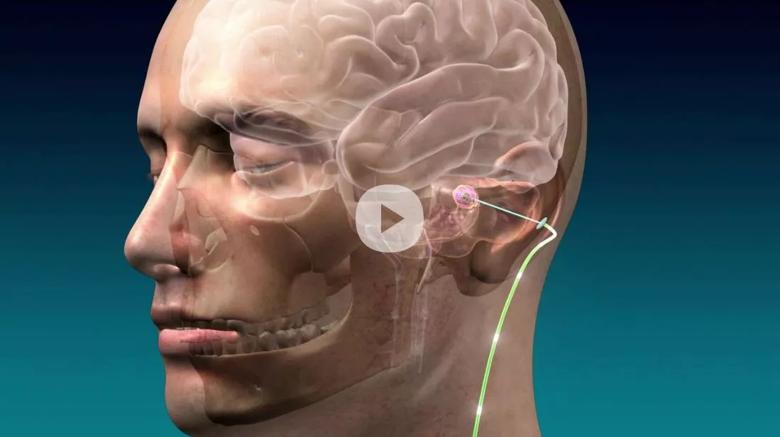
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/d52466e9-14e3-4b79-8411-a441d456c2ec/dbs2_jpg)
Click image for animation of DBS for post-stroke rehabilitation
A first-in-human trial of deep brain stimulation (DBS) for post-stroke rehabilitation patients by Cleveland Clinic researchers has shown that using DBS to target the dentate nucleus – which regulates fine-control of voluntary movements, cognition, language, and sensory functions in the brain – is safe and feasible.
The EDEN trial (Electrical Stimulation of the Dentate Nucleus for Upper Extremity Hemiparesis Due to Ischemic Stroke) also shows that the majority of participants (nine out of 12) demonstrated improvements in both motor impairment and function. Importantly, the study found that participants with at least minimal preservation of distal motor function at enrollment showed gains that almost tripled their initial scores.
Published in Nature Medicine, these findings build on more than a decade of preclinical work led by principal investigators Andre Machado, MD, PhD, and Kenneth Baker, PhD, at Cleveland Clinic.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/6b66e5bb-619a-4c58-b9e0-a6c823c9e6d7/NEU_4028388_06-30-23_1064_AMO-scaled_jpg)
Andre Machado, MD, PhD, (left) and Kenneth Baker, PhD
“These are reassuring for patients as the participants in the study had been disabled for more than a year and, in some cases, three years after stroke. This gives us a potential opportunity for much needed improvements in rehabilitation in the chronic phases of stroke recovery,” said Dr. Machado, chair of Cleveland Clinic’s Neurological Institute. “The quality-of-life implications for study participants who responded to therapy have been significant.”
Dr. Machado patented the DBS method in stroke recovery. Boston Scientific owns a license to those patents and provided the Vercise DBS systems used in the trial. In 2010, Cleveland Clinic Innovations established Enspire DBS Therapy, Inc., a Cleveland Clinic portfolio company and is commercializing technology developed at Cleveland Clinic to commercialize the method and it co-funded the study. Dr. Machado holds stock options and equity ownership rights with Enspire and serves as the chief scientific officer.
“We saw patients in the study regain levels of function and independence they did not have before enrolling in the research,” Dr. Machado said. “This was a smaller study and we look forward to expanding as we have begun the next phase.”
The completed EDEN trial enrolled 12 individuals with chronic, moderate-to-severe hemiparesis of the upper extremity as a result of a unilateral middle cerebral artery stroke 12-to-36 months prior. There were no major complications throughout the study. Nine of the 12 participants improved to a degree that is considered meaningful in stroke rehabilitation.
Each participant underwent DBS surgery, which involved the surgical implantation of electrodes into a part of the brain called the cerebellum. Once connected to a pace-maker-like device, the electrodes were used to deliver small electric pulses to help people recover control of their movements. Following discharge and recovery from the surgery, participants completed months of physical therapy, first with the DBS device turned off for several weeks and then turned on for four-to-eight months. It was after turning the device on that the most significant improvements were observed.
“The safety and feasibility data from this early study combined with the potential symptom improvements certainly support the need for additional, larger trials to see if cerebellar DBS is indeed a potential treatment for post-stroke motor impairment,” said Brooks Gross, PhD, program director, National Institute of Neurological Disorders and Stroke.
Stroke is the leading cause of long-term disabilities. Approximately 800,000 people in the United States alone suffer strokes every year. While the majority of patients will survive the acute phase, persistent neurological issues likely will jeopardize quality of life and productivity, with approximately 50% of survivors still exhibiting disability severities that require assistance with daily activities.
“There are currently no effective methods to improve the outcomes of physical rehabilitation for the hundreds of thousands of stroke survivors,” said Dr. Baker, Cleveland Clinic Lerner Research Institute. “The results of the study found that deep brain stimulation, paired with physical therapy, improved movement in patients who were more than a year out from their stroke and whose motor improvements had largely plateaued. This tells us the research warrants further investigation in larger patient samples.”
Research reported in this press release was supported by the NIH Brain Research Through Advancing Innovative Neurotechnologies® Initiative, or The BRAIN Initiative®, under award number UH3NS100543. The study was also supported by Enspire DBS Therapy, Inc.
Video content: This video is available to watch online.
View video online (https://www.youtube.com/embed/zNlA3gzZrWQ?feature=oembed&wmode=transparent)
Deep Brain Stimulation is Promising for Stroke Patients, Study Shows