Research aims to improve outcomes for ACL tears, a common orthopaedic injury
Cleveland Clinic News Service | 216.444.0141
We’re available to shoot custom interviews & b-roll for media outlets upon request.
CCNS health and medical content is consumer-friendly, professional broadcast quality (available in HD), and available to media outlets each day.


Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/c3640e7a-4ac6-4169-af8c-6aba81dfc3a8/spindler-081437_03-13-14_060_YKL-scaled_jpg)
Kurt Spindler, M.D.
The National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health (NIH), has awarded Cleveland Clinic $6 million to study techniques used for anterior cruciate ligament (ACL) reconstruction.
The five-year grant – led by principal investigator Kurt P. Spindler, M.D., of Cleveland Clinic and founder of the Multicenter Orthopaedic Outcomes Network (MOON) Group – supports a multi-center, randomized clinical trial aimed at determining if outcomes of a new surgical technique, Bridge-Enhanced® ACL Repair (BEAR®), are equal to or better than outcomes of traditional ACL reconstruction surgery. The Cleveland Clinic-led research will coordinate a consortium of five sites involved in this BEAR MOON trial. Other sites include The Ohio State University, Washington University St. Louis, Vanderbilt University, and Rhode Island Hospital (Brown University).
The current gold standard surgical treatment — autograft ACL reconstruction — stabilizes the knee, but has a number of drawbacks.
“The current standard for ACL surgery is a complex reconstruction procedure that has a high rate of success in terms of return to sports and activities of daily living. But the failure rate is high in adolescents.” Dr. Spindler said. “There is some graft site morbidity and the propensity to develop early posttraumatic osteoarthritis (PTOA) is not prevented.”
This research will build upon prior studies led by Martha Murray, M.D., at Boston Children’s Hospital, that showed the BEAR technique to have similar results to ACL reconstruction in preclinical and early clinical studies.
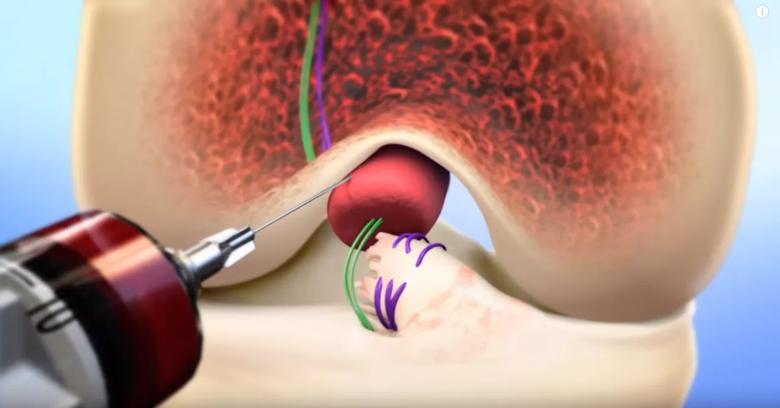
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/a3d6d28d-f11d-4dc9-9228-6ea5fa40edc5/ACL-V4_png)
BEAR® implant is placed between torn ACL ends. Blood drawn from the patient is added. Torn ACL ends are then pulled into implant with stitches. (Photo credit: Boston Children’s Hospital)
“In the BEAR procedure, we are not just stitching the ACL together,” Dr. Spindler said. “The magic, the key to making it work, is the scaffold.”
The BEAR procedure begins with drilling small tunnels in order to place a suture into the ACL fibers and to stabilize the knee. The tissue-engineered scaffold, invented by Dr. Murray, is implanted through a small incision in the knee. Surgeons then pull the stitched ACL tibial stump into the scaffold as the knee is extended. The patient’s own blood is applied to the scaffold to provide growth factors and stimulate healing. Patients are expected to return to normal activities in a few months and to sports in about nine months, Dr. Spindler notes.
Through this grant, the team will enroll 200 participants who are between 18 and 40 years of age and have complete ACL tears. Surgery will need to take place within 50 days of injury. The primary goal of the study is to evaluate the outcomes of Bridge-Enhanced ACL Repair procedure versus the standard autograft patellar tendon reconstruction at six months, one year and two years after surgery. Researchers expect earlier improved range of motion and knee kinematics in the short-term and no graft harvest morbidity for the patients treated with Bridge-Enhanced ACL Repair. The BEAR implant is an investigational device and it is only available in FDA approved clinical trials.
“Our goal in this trial is to see if we can duplicate the earlier single-center study results on a multicenter and multisurgeon level,” Dr. Spindler said. “The findings from this trial will hopefully help to change the standard clinical practice of ACL surgery.”
Ellen McErlean, MSN, RN, FAHA, is coordinating this study. Contact her at mcerlee@ccf.org.
This research is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, under award number R01AR074131. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Cleveland Clinic is a nonprofit multispecialty academic medical center that integrates clinical and hospital care with research and education. Located in Cleveland, Ohio, it was founded in 1921 by four renowned physicians with a vision of providing outstanding patient care based upon the principles of cooperation, compassion and innovation. Cleveland Clinic has pioneered many medical breakthroughs, including coronary artery bypass surgery and the first face transplant in the United States. Cleveland Clinic is consistently recognized in the U.S. and throughout the world for its expertise and care. Among Cleveland Clinic’s 82,600 employees worldwide are more than 5,786 salaried physicians and researchers, and 20,700 registered nurses and advanced practice providers, representing 140 medical specialties and subspecialties. Cleveland Clinic is a 6,728-bed health system that includes a 173-acre main campus near downtown Cleveland, 23 hospitals, 280 outpatient facilities, including locations in northeast Ohio; Florida; Las Vegas, Nevada; Toronto, Canada; Abu Dhabi, UAE; and London, England. In 2024, there were 15.7 million outpatient encounters, 333,000 hospital admissions and observations, and 320,000 surgeries and procedures throughout Cleveland Clinic’s health system. Patients came for treatment from every state and 112 countries. Visit us at clevelandclinic.org. Follow us at x.com/CleClinicNews. News and resources are available at newsroom.clevelandclinic.org.
Editor’s Note: Cleveland Clinic News Service is available to provide broadcast-quality interviews and B-roll upon request.