Device integrates wireless communication between leadless pacing and defibrillator therapy

Cleveland Clinic has successfully implanted a leadless pacemaker defibrillator system in the world’s first two patients as part of a global clinical trial. The novel device combines the technology of a leadless pacemaker with a subcutaneous implantable cardioverter defibrillator, which aims to deliver treatment for both low and elevated heart rates.
Pacemakers provide electrical stimulation to regulate a patient’s heartbeat while a subcutaneous- ICD is implanted to constantly monitor the patient’s heart rhythm and protect against sudden cardiac arrest which occurs in more than 356,000 people each year in the United States alone.
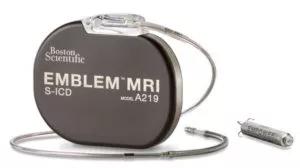
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/9f64ef15-bf29-4eeb-850b-9e5265ae2075/Image-courtesy-of-Boston-Scientific-2021-The-EMPOWER-MPS-is-an-investigational-device-and-limited-by-U-S-law-to-investigational-use-only-It-is-not-available-for-sale-300x168)
Image courtesy of Boston Scientific © 2021. The EMPOWER MPS is an investigational device and limited by U.S. law to investigational use only. It is not available for sale.
Unlike conventional pacemaker-ICD’s, this leadless system does not require wires to be threaded through the blood vessels, which are vulnerable to fracture over time and are associated with blood clot risks. In the past, patients receiving subcutaneous-ICD alone, did not have the capability to receive back-up pacing support for abnormally slow heart rhythms, including potentially lethal asystole, (cardiac flatline) and did not have the capability for painless termination of sudden cardiac arrest due to ventricular tachycardia, which is overwhelmingly the most common lethal arrhythmia.
“Modular therapy curates implantable medical device therapy to the specific needs of patients throughout their lifetime so that they receive only the device they need when they need it,” said Daniel Cantillon, M.D., research director and associate section head of Cardiac Electrophysiology and Pacing in the Heart, Vascular & Thoracic Institute at Cleveland Clinic and global principal investigator. Dr. Cantillon is a consultant for Boston Scientific.
“Combined use of both types of devices in a leadless approach could benefit a much larger patient population,” said Dr. Cantillon. “Furthermore, while life-saving, ICD shocks are both painful and psychologically traumatizing for patients. It’s our hope that the combination of a tiny leadless pacemaker implanted directly into the heart with the subcutaneous-ICD in the soft tissue will allow the majority of lethal arrhythmias to be painlessly terminated without long-term risks.”
The multi-center, non-randomized MODULAR ATP clinical trial- sponsored by Boston Scientific, manufacturer of the modular system- will enroll up to 300 patients across 50 centers in the U.S., Canada and Europe, including patients who require a new ICD or who already have a subcutaneous-ICD system implanted. The trial will evaluate the safety, performance and effectiveness of the modular cardiac rhythm management system.
Cleveland Clinic is a nonprofit multispecialty academic medical center that integrates clinical and hospital care with research and education. Located in Cleveland, Ohio, it was founded in 1921 by four renowned physicians with a vision of providing outstanding patient care based upon the principles of cooperation, compassion and innovation. Cleveland Clinic has pioneered many medical breakthroughs, including coronary artery bypass surgery and the first face transplant in the United States. Cleveland Clinic is consistently recognized in the U.S. and throughout the world for its expertise and care. Among Cleveland Clinic’s 82,600 employees worldwide are more than 5,786 salaried physicians and researchers, and 20,700 registered nurses and advanced practice providers, representing 140 medical specialties and subspecialties. Cleveland Clinic is a 6,728-bed health system that includes a 173-acre main campus near downtown Cleveland, 23 hospitals, 280 outpatient facilities, including locations in northeast Ohio; Florida; Las Vegas, Nevada; Toronto, Canada; Abu Dhabi, UAE; and London, England. In 2024, there were 15.7 million outpatient encounters, 333,000 hospital admissions and observations, and 320,000 surgeries and procedures throughout Cleveland Clinic’s health system. Patients came for treatment from every state and 112 countries. Visit us at clevelandclinic.org. Follow us at x.com/CleClinicNews. News and resources are available at newsroom.clevelandclinic.org.
Editor’s Note: Cleveland Clinic News Service is available to provide broadcast-quality interviews and B-roll upon request.